The first
heating mechanism is
caused by the molecules in a polar liquid “hooking
on” each
other due to the uneven charge distribution of the molecule, so that
one end gets an excess of positive charge and the other a
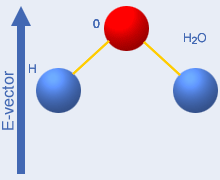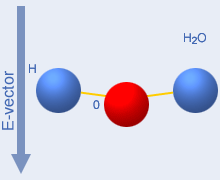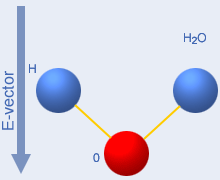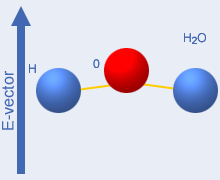
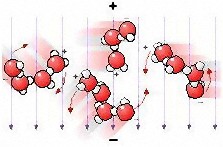
negative charge – see the picture to the right which shows
water
molecule groups. The microwave field (+ and – and
downwards
directed arrows in the picture) will then exert a turning force on the
molecules – they attempt to align with the field and energy
is
supplied to them. When the field direction changes (this occurs 4,9
billion times per second at 2450 MHz), the molecules return the
alignment
energy to the field –
the system behaves like an electric capacitor connected to an
alternating voltage. If the microwave frequency is very high, the
molecule groups will however rotate so sluggishly (in relative terms)
that they do not at all align with the field – no energy
transfer
takes place.
In a certain frequency interval, the molecule groups will
(statistically) still rotate but with some lag, which depends
on their mass
inertia. All energy will then not be recovered when the field direction
is reversed. A part will instead be permanently given off to the
molecule groups, which will heat up by a general net increase in their
movement. The phenomenon is called dipole
relaxation.
For water, the maximum absorption capability at room temperaure is at
about 20 000 MHz. The capability is
“suitable” at
2450 MHz, i.e. sucessive microwave absorption occurs down to about a
depth of 20 mm in materials
with a high water content. The capability also depends on the
temperature and decreases when it rises, since the number of molecules
hooking on to each other is reduced with increasing general, incessant
movement (so-called Brownian motion).
The two movie images below the top illustration shows what
does
not happen with microwaves. Simple rotation cannot occur due to the
high collision rate.The flexing shown to the right occurs in individual
molecules exposed to infrared radiation with 1…5
µm
wavelength, and is the cause of the blue colour of water. The left
image is from this excellent “water
site”, and the right from the Peter Püschner industrial microwave
site. |
|
The other
heating mechanism results from many liquids
(e.g. water) being able to dissolve salts, acids and
bases which then become dissociated into charged ions.The ions are
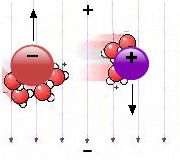
influenced by an electric field causing a net movement in its direction
– see the image to the right, which shows a negative ion
(e.g.
the chloride ion in common salt) and a positive ion (e.g. sodium).
That the ions get an additional net movement is equal to a temperature
increase, by the lectric energy being converted into heat. This effect
is
frequency independent.The ionic (or electrolytic) conductivity measured
at low frequency can directly be recalculated into a part of the
microwave absorption capability. This increases with increasing
temparature, since the shielding by adjacent water molecule groups
diminishes with increasing temperature – the ions
become
more “naked”.
The mechanism described here is called ionic conductivity. |
 |
| In
common food substances, the two mechanisms contribute about equally to
the overall microwave absorption capability, in spite of the salt
content typically being about 0,6 % or less. There is also a
favourable compensation by opposite temperature depencences of the two
mechanisms. – A diagram showing this: (high resolution PNG; simpler
PDF).
Other material data and a description of the
penetration depth concept. |
Read
here
about the two other heating mechanisms and why microwaves
“only” heat up substances. |